Authors: de Clifford, L., Vallance, S. A., Wood, E., Gieseg, M., Lowe, J.N.
Contact author: Leigh de Clifford | Leigh@matavet.co.nz
INTRODUCTION:
Management of joint-related conditions presents a significant challenge. This retrospective study evaluates the long-term safety of 2.5% iPAAG (Arthramid®) in racing Thoroughbreds.
METHODS:
Clinical records from a single Australian veterinary practice (2017–2023) were reviewed alongside searches of the Racing Australia website. Horses treated with intra-articular 2.5% iPAAG alone were included in the study. Name, joint treated, volume, Timeform rating, win %, and 1st-3rd % were recorded for each date of treatment. Efficacy was evaluated using performance Timeform ratings only in horses with multiple joints treated or multiple treatments in the same joint. Adverse events and catastrophic musculoskeletal injuries (CMIs) were recorded and treatment intervals analysed. Descriptive statistical analysis in Microsoft Excel (CI 90. P<0.05).
RESULTS:
Safety was assessed in 701 TB racehorses aged 2-9 years (mean 4.2) across 15,050 race starts receiving 2,312 intra-articular injections. Mean time to race following first treatment was 40 days (CI: 25,55). Total of three adverse events were reported (0.13% of injections), and the CMI rate (3 in total) was 0.199 per 1,000 starts.
205 horses had multiple joints treated or multiple treatments in the same joint (Table 1.1 and 1.2). In this group the most treated joints were middle carpal (262/ 39%), front fetlock (219/33%), hind fetlock (73/10.9%), and tarsometatarsal (64/ 9.5%) (Table 1.3) Most common treatment volume was 2mL (50mg) (Table 1.4). Mean treatment interval was 234 days (Table 1.5).
Performance analysis showed pre-treatment Timeform ratings (mean 71.7) significantly improved after initial treatment and this improvement was maintained, regardless of the joint or how many treatments were given (Figure 1). There was no significant change in win or placing (1st to 3rd) percentages. There was no effect of number of treatments on number of starts (average of 28), nor number of days in training (average of 1100). At study conclusion, 78 (38.0%) of horses remained in active training, 123 (60.0%) had retired for non-joint related reasons (e.g. breeding, poor performance, respiratory issues, other). Four horses (1.9%) retired due to ongoing issues with the treated joint.
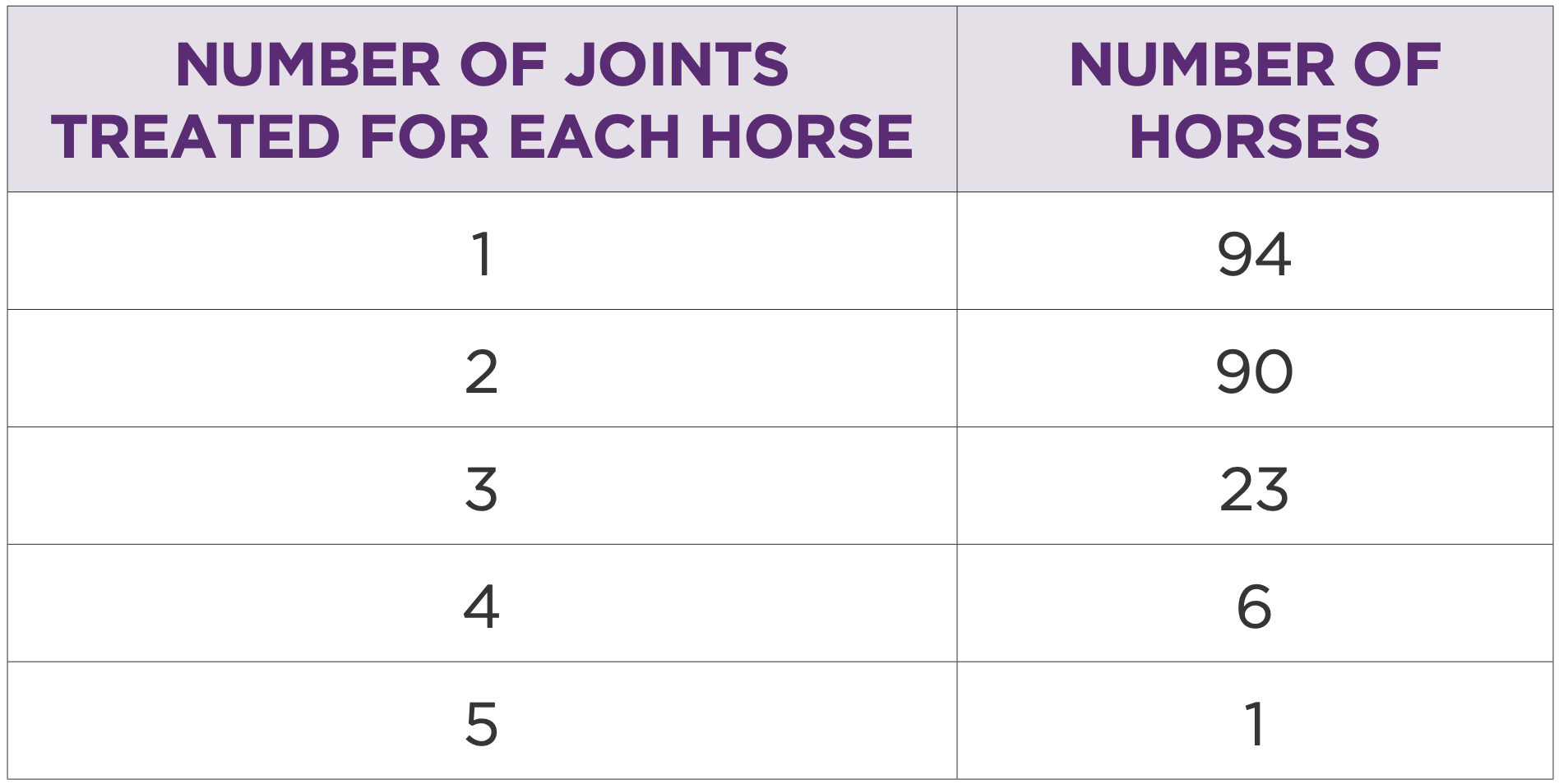
Table 1: Number of horses by number of joints treated
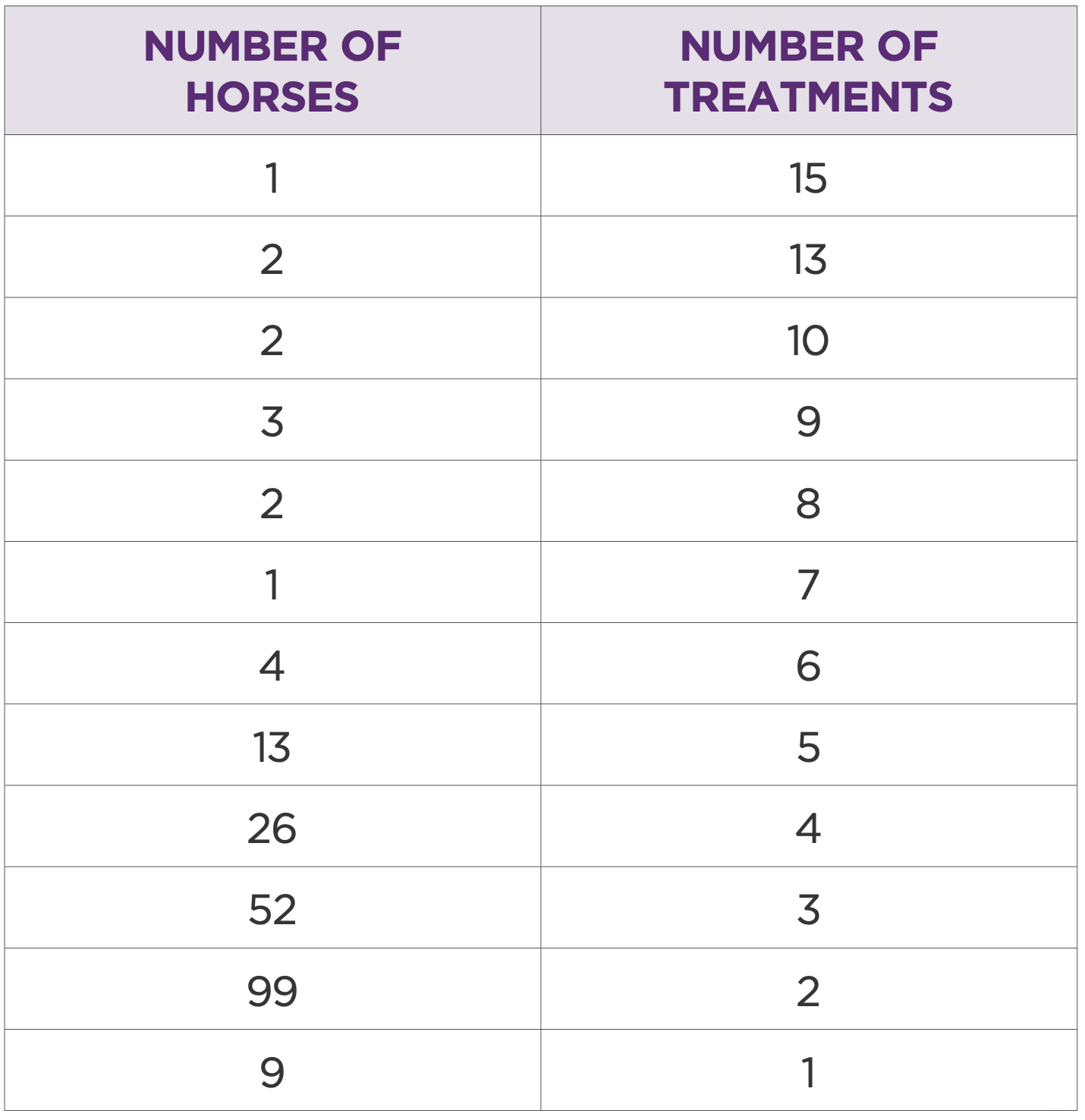
Table 2: Number of horses by number of treatments for each horse
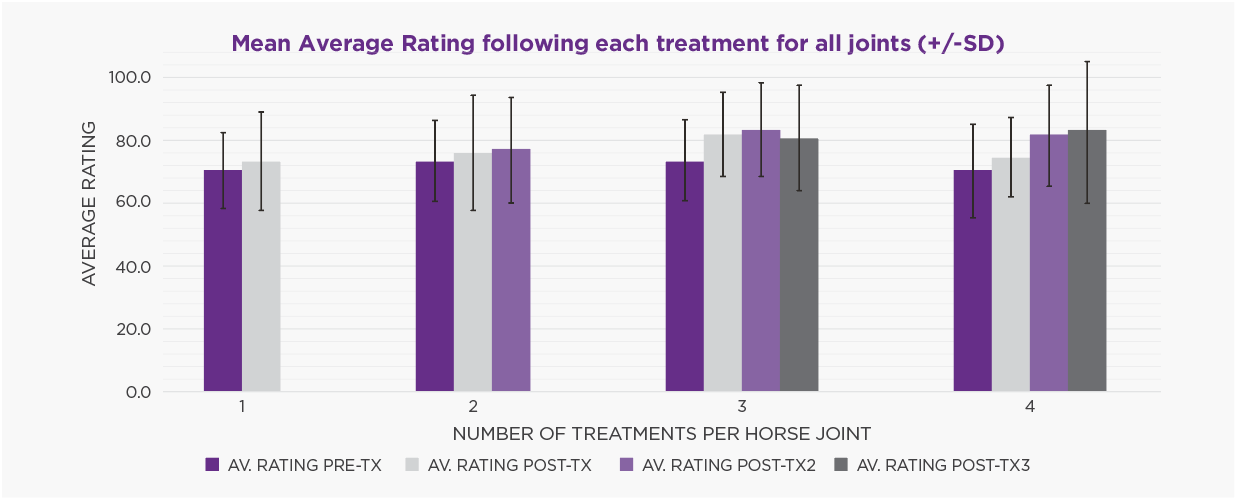
DISCUSSION:
This study provides compelling evidence for the safety and clinical utility of 2.5% iPAAG in managing joint disease in high-performance equine athletes. The exceptionally low CMI rate —substantially lower than the Australian industry standard of 0.52/1,000 (1) and minimal adverse events- 0.13% compared to published rates of up to 2% (2)- highlight the product’s favourable safety profile, even with repeated administration.
The extended treatment interval suggests a durable therapeutic effect, reducing the need for frequent intra-articular interventions (3). Performance data indicates that 2.5% iPAAG allows the horse to perform to the best of its ability.
Career longevity data, the percentage of horses remaining in active training (38.0%) or retiring for non-joint related reasons (60.0%) further supports the long-term safety profile. The low rate of joint-related retirement (1.9%) is particularly noteworthy.
Conclusion: This is the largest safety and efficacy study of 2.5% iPAAG in Thoroughbred racehorses to date. Despite the inherent limitations of retrospective analysis (potential selection bias and lack of controls) the results support its use as a safe, effective, and welfare-conscious option for long-term joint management in racing horses.
References:
1. Whitton RC, Jackson MA, Campbell AJD, et al. (2014). Musculoskeletal injury rates in Thoroughbred racehorses following local corticosteroid injection. The Veterinary Journal 2014; 200(1):71-76.
2. Kynch H, Vidal M, Chouicha N et al. Cytokine, catabolic enzyme and structural matrix gene expression in synovial fluid following intra- articular administration of triamcinolone acetonide in exercised horses. Equine Vet J 2017; 49:107-115.
3. Tnibar A. Review of intra-articular 2.5% polyacrylamide hydrogel, a new concept in the management of osteoarthritis. Eq Vet Education 2024;37: 161-168.
PUTTING IT INTO PRACTICE:
Arthramid® is indicated for use in any synovial joint exhibiting clinical signs of osteoarthritis, including but not limited to joint pain, synovitis, effusion, pain on flexion, and lameness responsive to intra-articular analgesia. It is also appropriate where diagnostic imaging (such as radiography, ultrasonography, scintigraphy, CT, or MRI) reveals abnormal joint pathology consistent with OA.
Early intervention is recommended, particularly in cases presenting with synovitis and capsular thickening, although Arthramid® remains effective in chronic and severe presentations of osteoarthritis.
Prior to administration, a thorough review of the patient’s history is essential to rule out soft tissue pathology, active infection, or suspected fracture in order to minimise the risk of complications and ensure appropriate use.
The dose of 2.5% iPAAG may vary depending on the severity of disease, the size of the joint and duration of clinical signs. The following recommendations have been made based on observed clinical responses to treatment as seen in this and other studies.
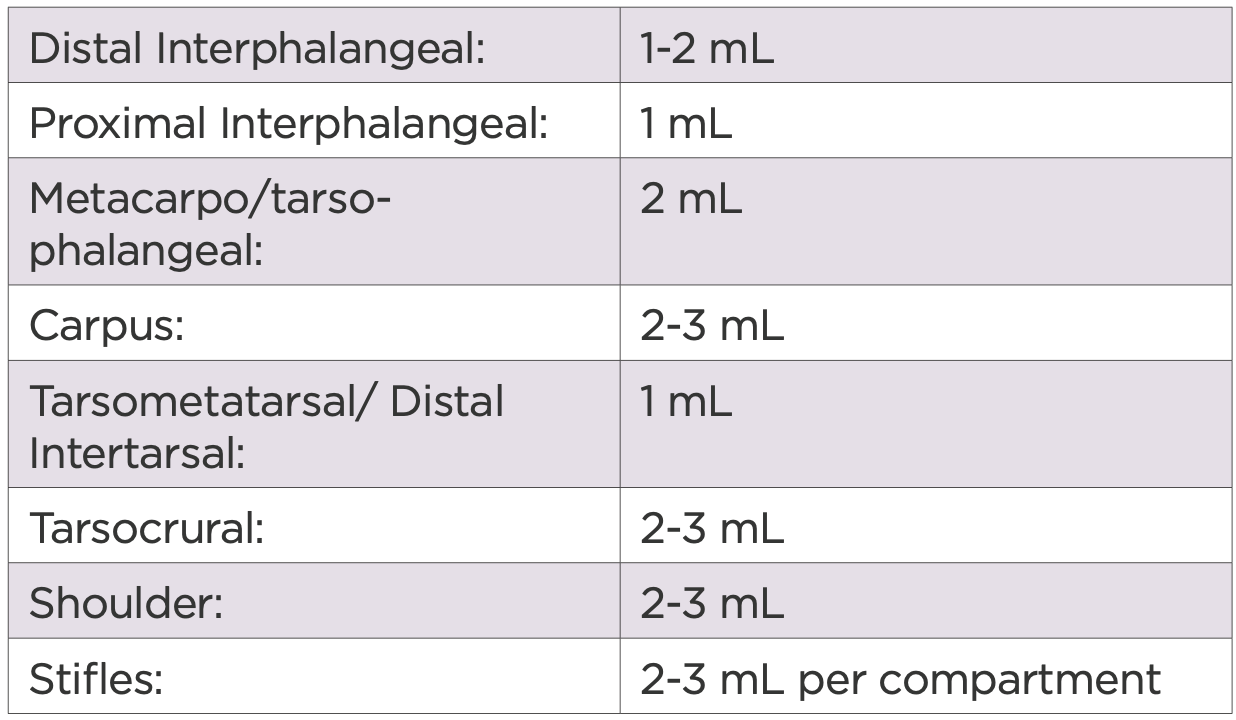
Table 3: Dose recommendations by joint

