Authors: Sandra Kurras DVM1, Marilene Appelhoff DVM1, Marc Koene DVM1
INTRODUCTION:
Joint disease remains a significant cause of reduced performance and early retirement in sport horses.
While various intra-articular treatment options exist, 2.5% iPAAG has emerged as a notable treatment option for veterinarians with its unique viscoelastic properties that restores synovial membrane bio-mechanics and potential for long term efficacy.
OBJECTIVE:
To evaluate the long-term safety of 2.5% injectable polyacrylamide hydrogel (2.5% iPAAG) in a large population of sport horses and assess its efficacy in treating joint-related lameness.
MATERIALS AND METHODS:
Medical records of 1000 horses treated with 2.5% iPAAG between November 2010 and December 2022 were analysed. Safety assessment included all 1000 horses, while efficacy analysis was conducted on 252 horses with complete follow-up data.
Pre-treatment lameness was scored using the AAEP scale. Treatment outcomes were evaluated through medical records and owner surveys.
RESULTS:
The study population comprised 83% warmbloods with a mean age of 11 years (range 3-28). The most commonly treated joints were coffin (40%), fetlock (32%), pastern (9%), and stifle (7%).
One adverse event was reported (0.1%) and determined unrelated to 2.5% iPAAG treatment.
Of the 252 horses with complete follow-up, 74.2% showed significant improvement, 70.0% returned to previous performance levels, and 77.0% remained actively competing.
The treatment demonstrated particular efficacy in chronic cases, with a low re-treatment rate of 6.8% in the same joint.
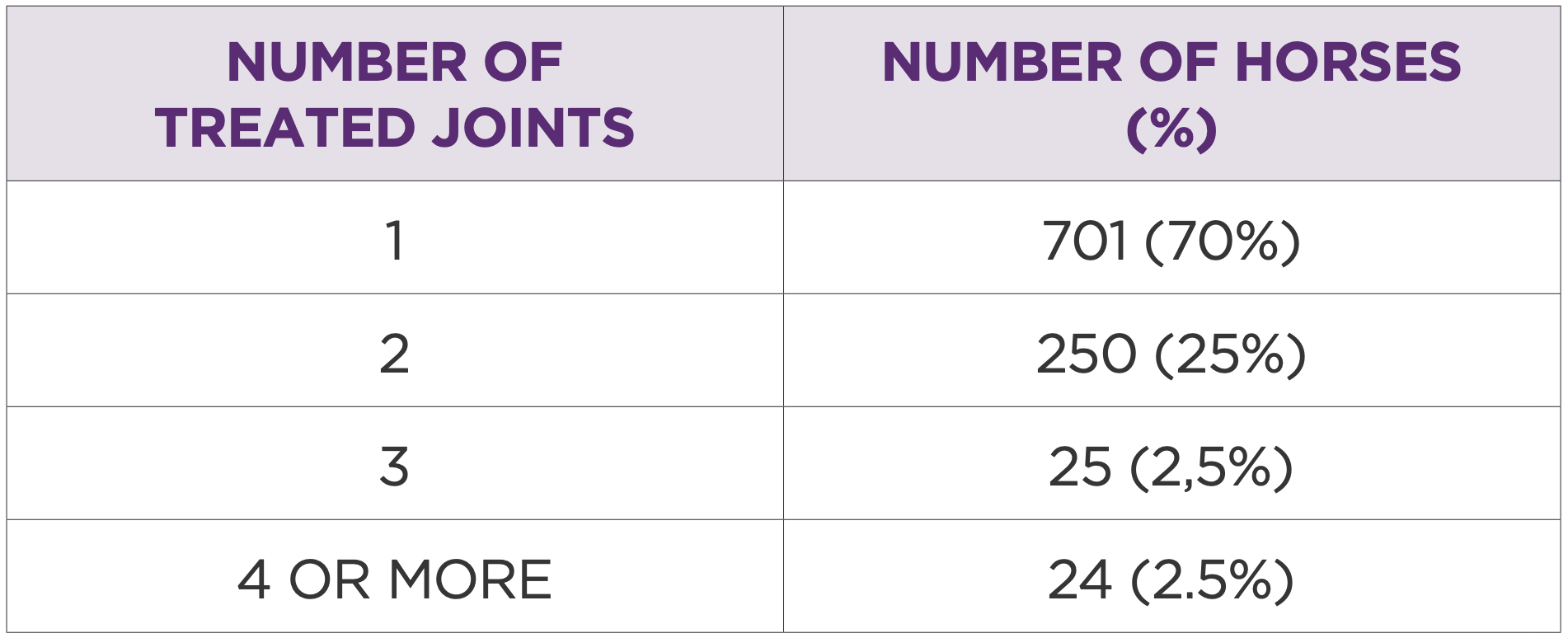
Table 1: Number of horses by number of joints treated
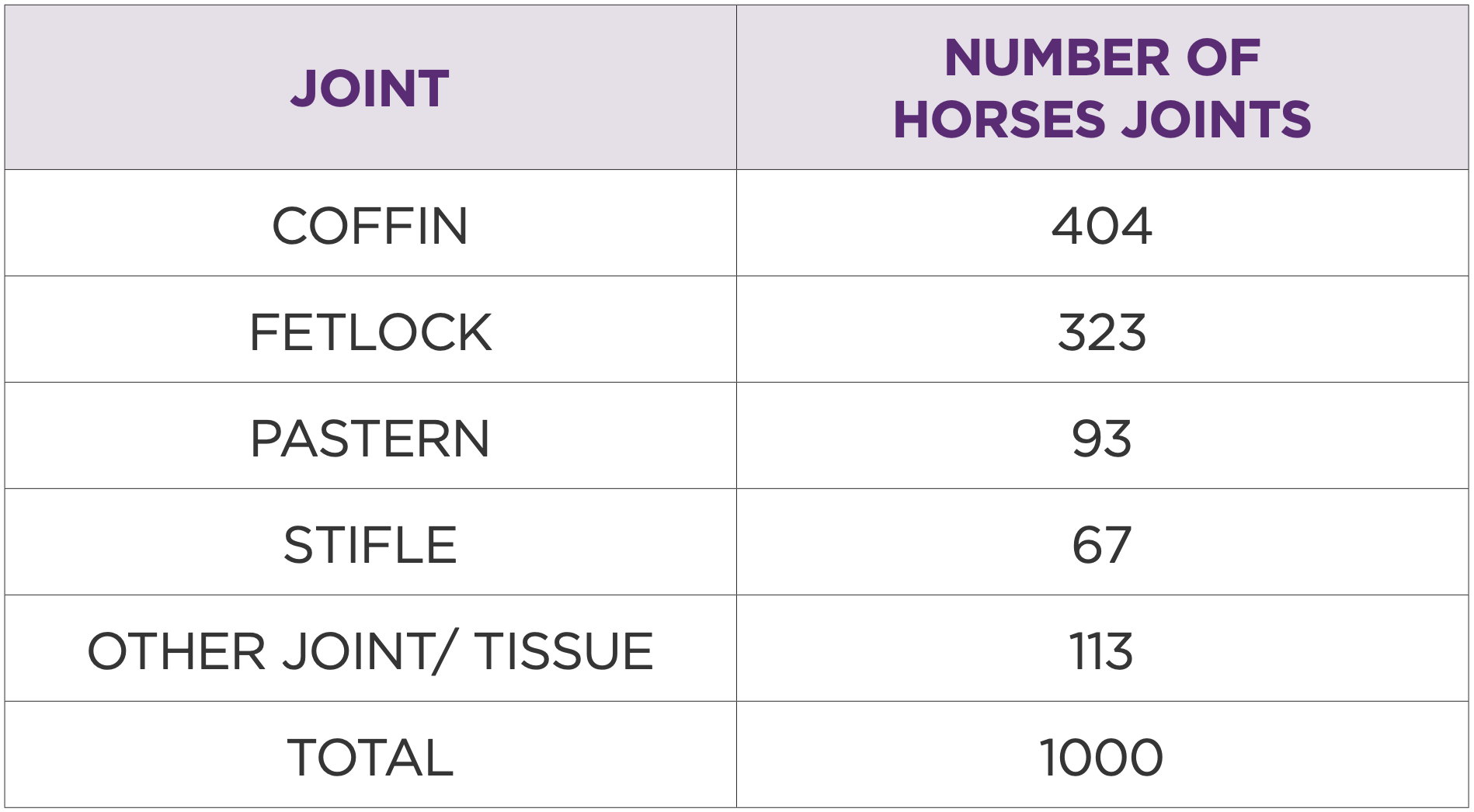
Table 2: Type of joints treated and the total number of treatments
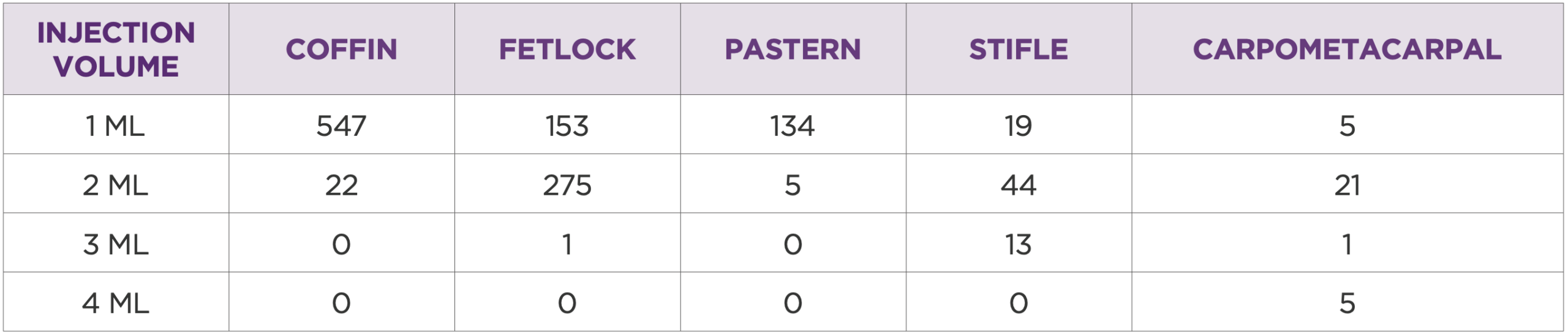
Table 3: Injection volume for the five most treated joints
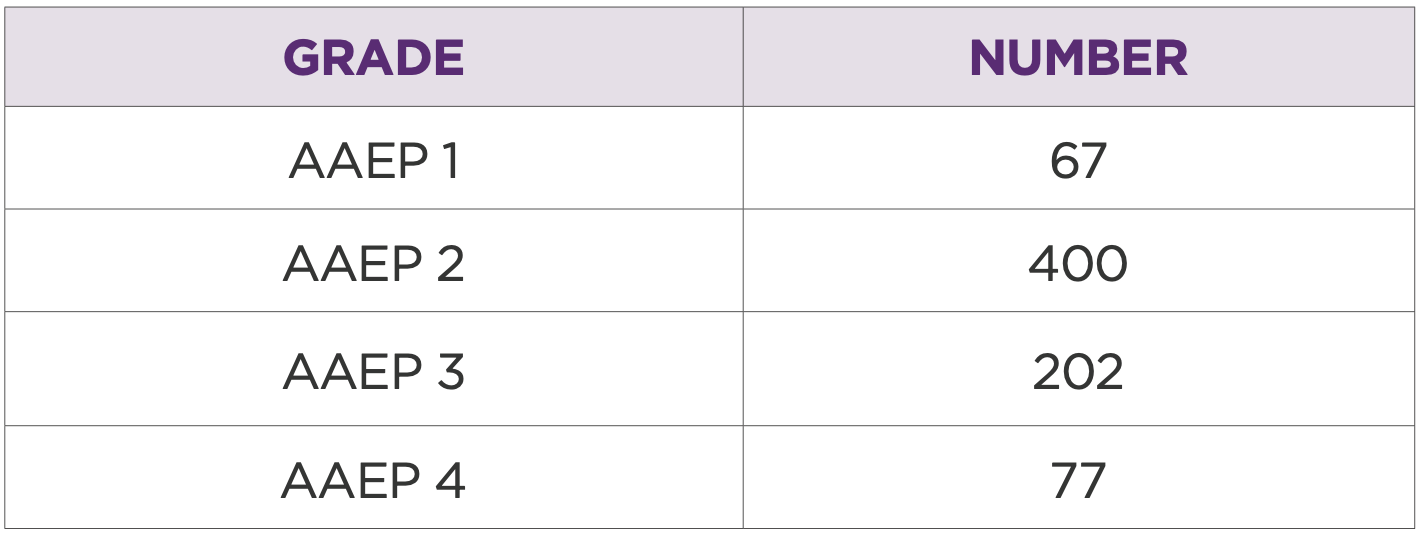
Table 4: Distribution of horses based on AAEP grade prior to injection
CONCLUSIONS:
This large-scale study demonstrates that 2.5% iPAAG is safe and effective for treating joint-related lameness in sport horses, with minimal complications and high return-to-performance rates.
The results support its use as a viable long-term treatment option in equine practice. The multimodal approach employed likely contributed to positive outcomes, highlighting the importance of comprehensive case management.
The distribution of treated joints reflects common sites of performance-limiting pathology in sport horses, with distal limb joints predominating. The successful treatment of these high-motion joints suggests broad applicability of 2.5% iPAAG across different anatomical locations.
PUTTING IT INTO PRACTICE:
In the study population, 2.5% iPAAG was administered as part of a multi-modal treatment approach designed to enhance therapeutic efficacy. This integrated strategy helps address multiple aspects of joint health and improve overall outcomes for horses.
As with any intra-articular therapy it is recommended to rest the horse for 48 hours immediately after treatment and then ideally plan a reduced impact workload for up to 2 to 4 weeks to allow full integration of the 2.5% iPAAG to occur. A follow up examination at 4 to 6 weeks is advised to assess the response to treatment.
Horse’s typically show a gradual reduction in lameness in the first week after treatment and this should continue to improve over the ensuing weeks. Repeated doses of Arthramid® can be safely given if clinically indicated.
The dose of 2.5% iPAAG may vary depending on the severity of disease, the size of the joint and duration of clinical signs. The following recommendations have been made based on observed clinical responses to treatment as seen in this and other studies.
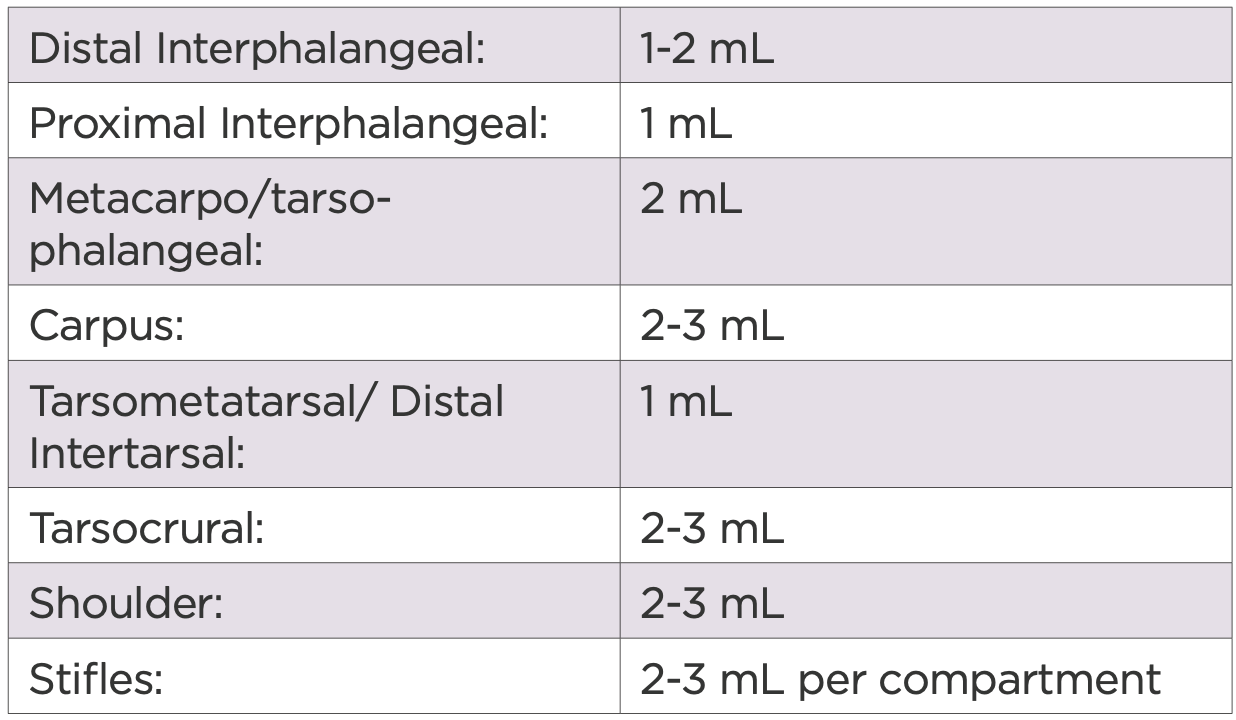
Table 5: Dose recommendations by joint
References
1. Tierklinik Lüsche GmbH, Lüsche, Germany

