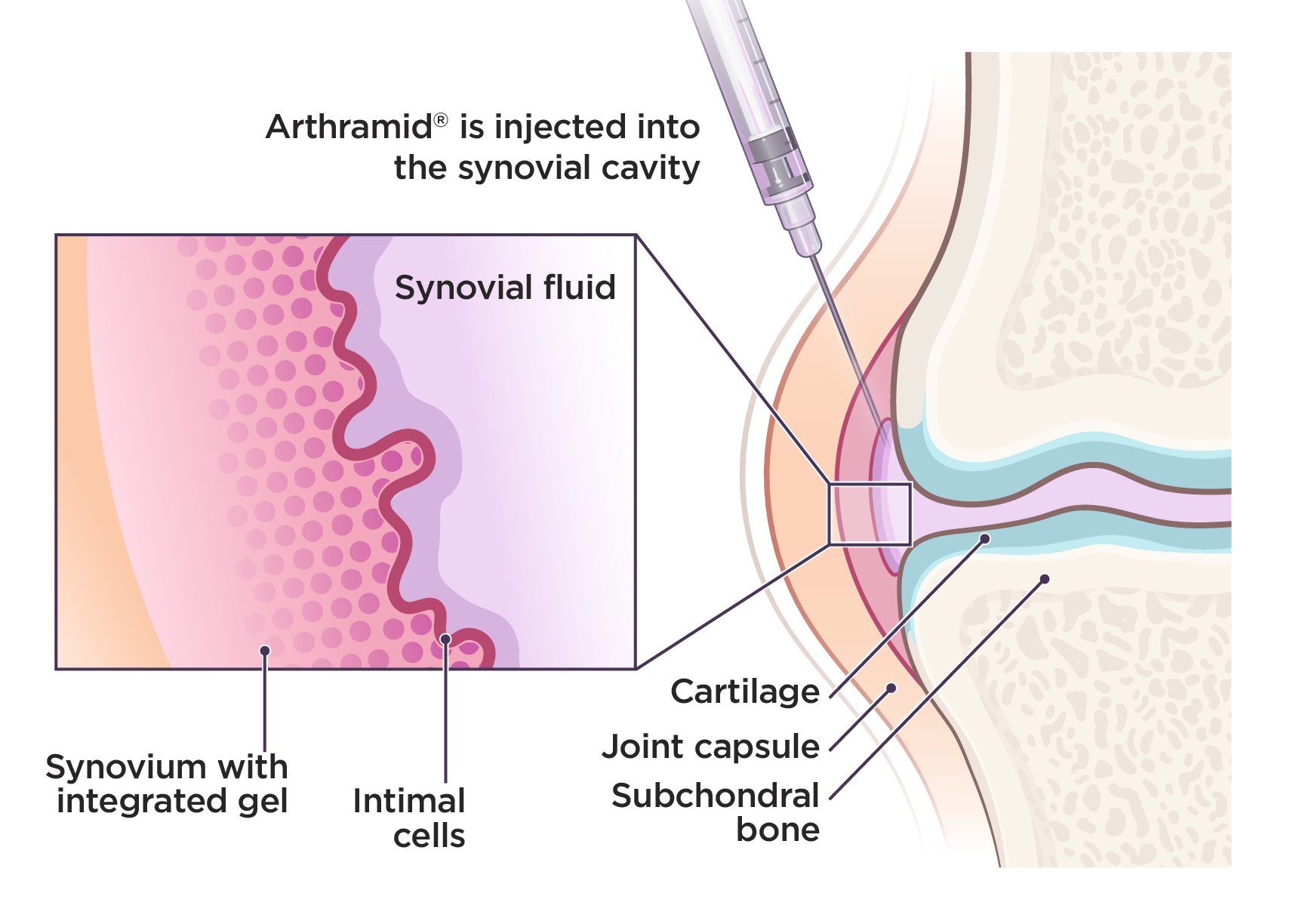
48 HOURS OF REST
As with any intra-articular injections, 48 hours of rest is advised. Occasionally, some dogs may experience local pain and/or mild edema at the injection site. NSAIDs and ice can be used to manage any discomfort. This is a transient response and should resolve within 2-3 days.
WEEKS CONTROLLED EXERCISE
Remembering the structural mechanism of action, it is important to maintain calm, controlled exercise during the initial product integration period. Leash walks, gradually increasing in length, are permitted. Rough play, running, and jumping should be avoided.
CONTINUE REHAB
Patients currently receiving rehabilitation or physiotherapy should continue their program. However, avoiding additional strain on treated joints is still advised for at least 2 weeks following treatment.
CONCURRENT REHAB MODALITIES
Based on currently available data, we do not recommend the use of shockwave therapy on the treated joint for a period of 6 weeks post injection. Neither do we recommend the use of laser therapy on or over the treated joint for a period of 2 weeks.
RETURN TO NORMAL ACTIVITY
“Normal activity level” will vary for each patient. It is important to consider each patient’s baseline activity level prior to treatment. Overexertion and low fitness can lead to secondary injury. Age, weight, and severity of disease may all impact patient’s return to increased activity.
RECHECKS AND REINJECTION
Rechecks are recommended at 6 to 8 weeks post-treatment. While a single treatment is often adequate, a small number of cases may benefit from a top-up dose. In cases that have not responded, it is important to reassess the accuracy of the diagnosis