Authors: Sandra Kurras DVM1, Marilene Appelhoff DVM1, Marc Koene DVM1
INTRODUCTION:
Joint-related lameness is a leading cause of reduced performance and premature retirement in sport horses.
Among available intra-articular therapies, 2.5% injectable polyacrylamide hydrogel (iPAAG; Arthramid®) has gained attention for its unique viscoelastic properties, which support synovial membrane biomechanics and offer sustained therapeutic effects. Despite its increasing use, long-term safety data remain limited.
OBJECTIVE:
To evaluate the long-term safety and efficacy of 2.5% iPAAG in the treatment of joint-related lameness.
MATERIALS AND METHODS:
Medical records of 1,000 horses treated with 2.5% iPAAG at a single veterinary practice in Germany between November 2010 and December 2022 were analysed. Safety was assessed across all cases, while efficacy was evaluated in 252 horses with complete follow-up data obtained from medical records, owner surveys, and online databases (pending).
Pre-treatment lameness was scored using the AAEP scale. Treatment date, joint treated, dose, diagnosis, ancillary treatments, discharge outcomes, and follow-up examination results were recorded in Microsoft Excel 365 and analysed using summary statistics (CI 95%, p<0.05).
RESULTS:
The study population consisted of 83% warmbloods (830 horses) and 17% other breeds, with a mean age of 11 years (range 3–28). All horses underwent diagnostic analgesia and radiographic or advanced imaging.
The most commonly treated joints were the coffin (40%), fetlock (32%), pastern (9%), and stifle (7%). One adverse event (0.1%) was reported and deemed unrelated to iPAAG treatment.
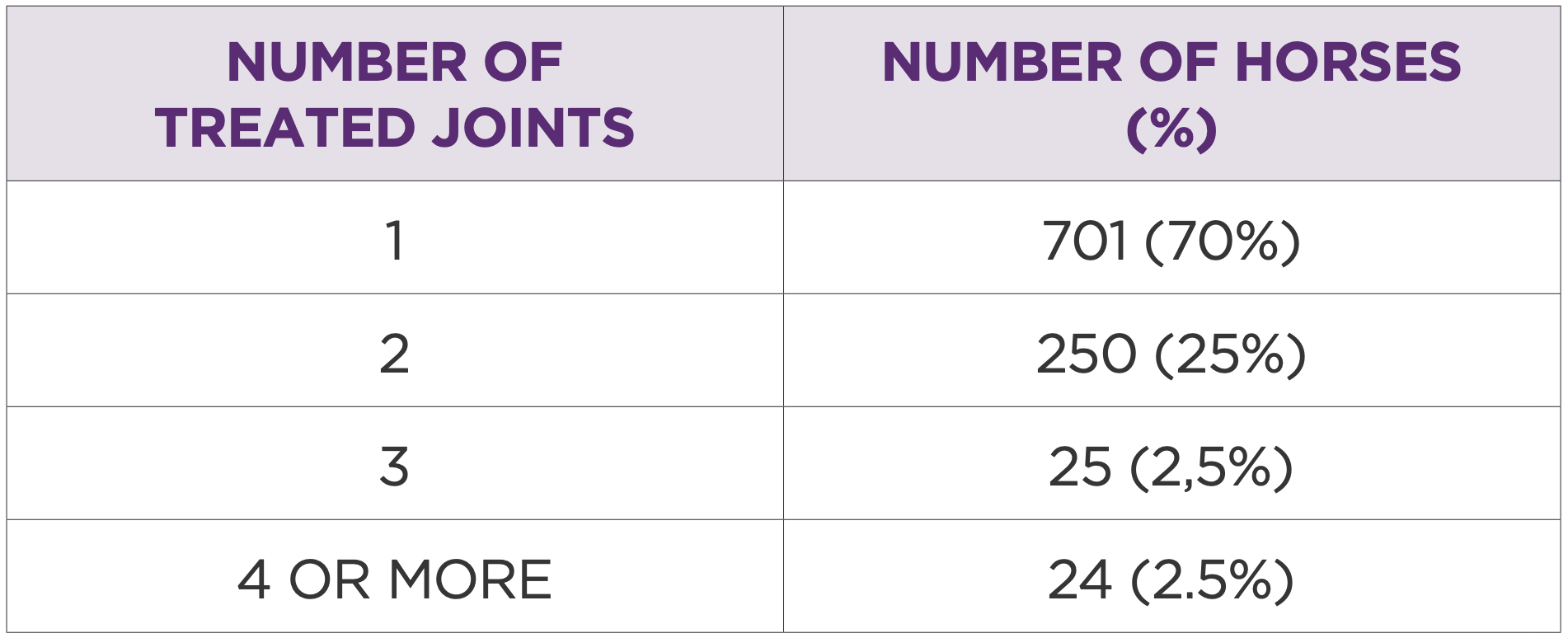
Table 1: Number of horses by number of joints treated
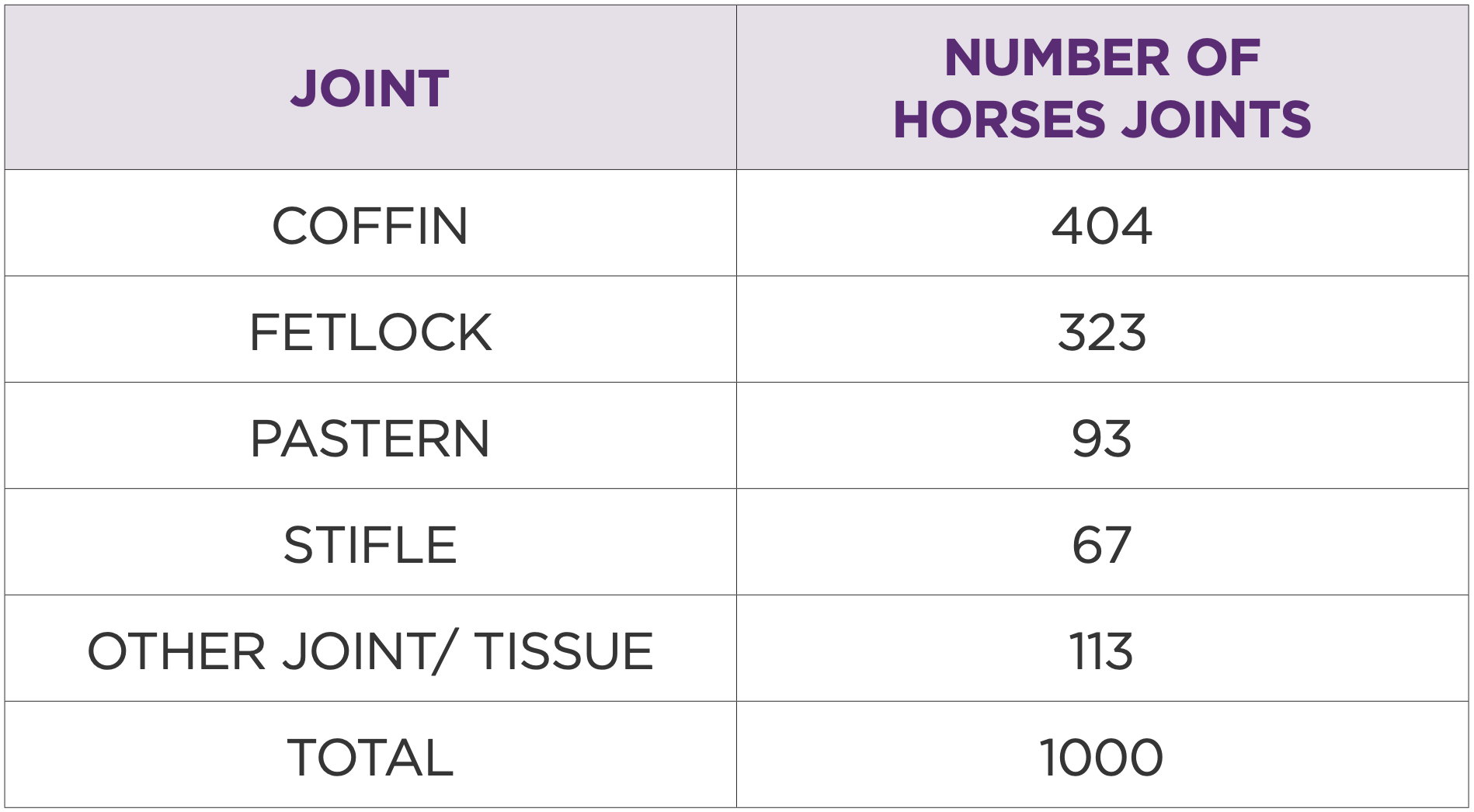
Table 2: Joints treated by total number of horses
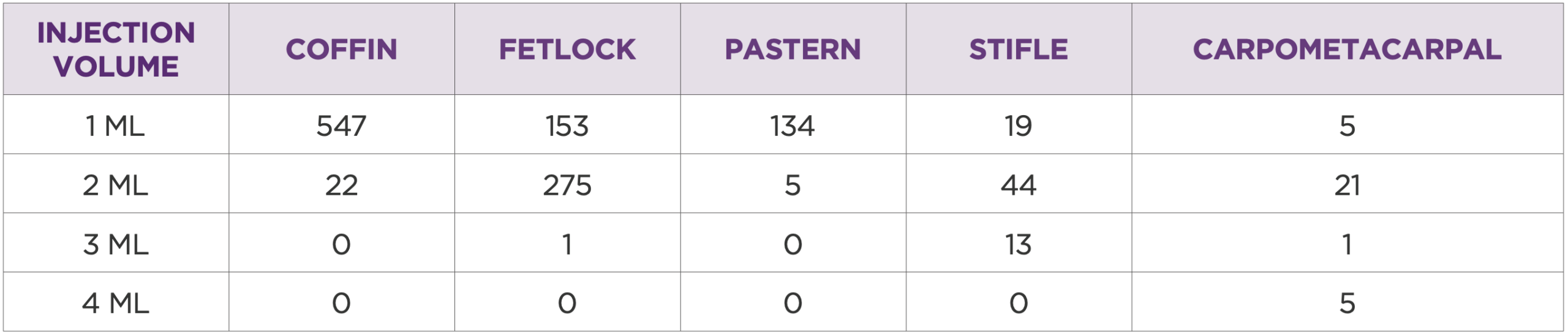
Table 3: Injection volume by joint
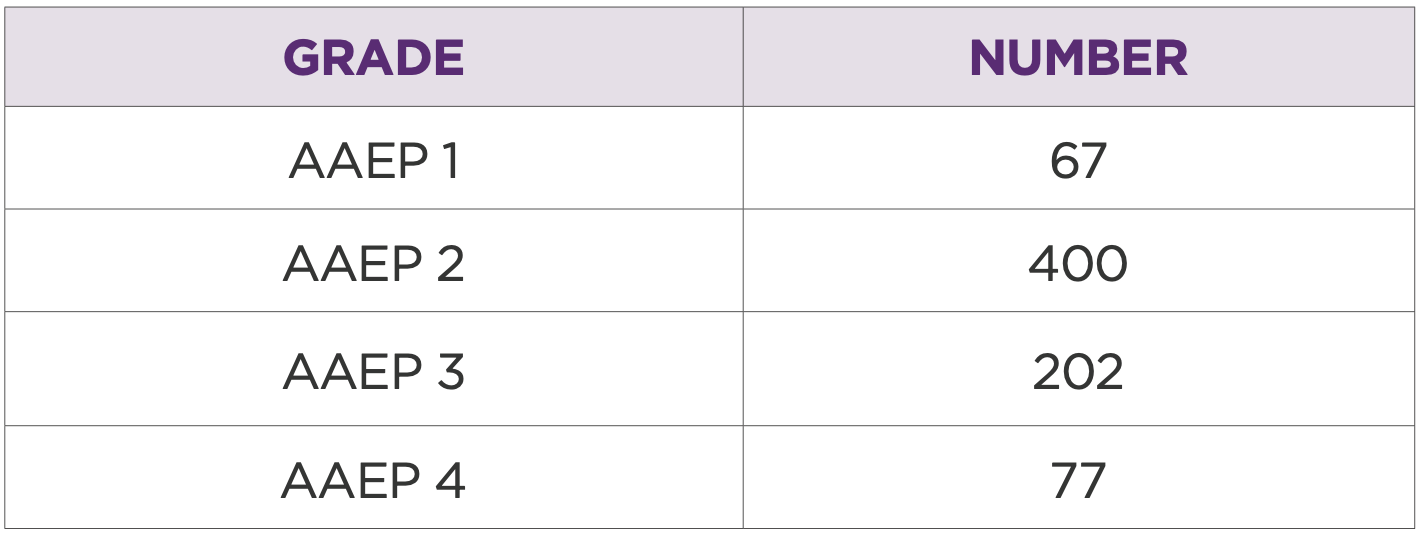
Table 4: Distribution of horses based on AAEP grade prior to treatment
The most frequent underlying conditions included osteoarthritis, osteoarthrosis, sclerosis, cartilage erosion and degradation, synovitis, and capsulitis.
Corrective shoeing was performed in 600 horses, and rehabilitation protocols were prescribed at discharge in 950 cases. Of the total, 70% (701 horses) had one joint treated, while 30% (299 horses) had two or more joints treated (Table 1).
The most treated joints were DIP (coffin) 404/40.4%, fetlock 323/32.3%, pastern 93/9.3%, stifle 67/6.7%, and other joints 113/11.3% (Table 2). Injection volumes were typically 1 ml or 2 ml, depending on the joint (Table 3).
AAEP scores were recorded for 746 horses, with an average score of 2.3 (Table 4).
2.5% iPAAG was administered as part of a multimodal treatment approach. Concurrent therapies were used in 389 horses (38.9%), including bisphosphonates (115/11.5%), calcium dobesilate (20/2.0%), NSAIDs (46/4.6%), intra-articular corticosteroids (154/15.4%), and orthobiologics (PRP, IRAP) (54/5.4%).
The single adverse event was associated with screw fixation of a fractured pastern and was considered unrelated to iPAAG treatment.
Of the 1,000 horses, 252 owners participated in a long-term follow-up survey.
- 187 (74.2%) reported significant improvement
- 36 (14.3%) reported some improvement
- 156 (70.0%) reported return to the same or better performance level
- 37 (16.6%) reported near return to previous performance
- 194 (77%) remained actively competing
- 46 (18.3%) retired due to joint-related issues
- 12 (4.7%) retired or were euthanised for non-joint-related reasons
DISCUSSION:
This large-scale study demonstrates that 2.5% iPAAG is a safe and effective treatment for joint-related lameness in sport horses, with minimal complications and high return-to-performance rates. All horses received mepivacaine prior to iPAAG administration, which may help mitigate non-septic inflammatory reactions (Personal Communication).
Long-term follow-up revealed no fibrosis, joint thickening, stiffness, or increased injury risk, supporting the strong safety profile of 2.5% iPAAG. The multimodal treatment approach likely enhanced therapeutic outcomes. A standardised rehabilitation protocol (2 weeks walking, 2 weeks walking and light trotting, 2 weeks trotting) was used in most cases, aligning with the known integration period of iPAAG into sub-synovial tissue. Treating early in the competition season or during low activity periods may further improve outcomes.
Dosage recommendations were based on clinical response and varied by joint size, disease severity, and duration of clinical signs. Common volumes included 1 ml for DIP and pastern joints, and 2 ml for fetlock, middle carpal, and stifle joints (per compartment).
Study limitations include lack of controls, potential owner bias, and the retrospective nature of the data. However, the large sample size, consistent outcomes across multiple joints, and standardised protocols support the long-term safety and efficacy of 2.5% iPAAG. While the multimodal approach may confound treatment effects, it reflects real-world practice and underscores the importance of comprehensive case management, especially with coffin joint lame. The distribution of treated joints mirrors common performance-limiting pathologies in sport horses, with distal limb joints predominating. Successful treatment of these high-motion joints suggests broad applicability of 2.5% iPAAG for equine practitioners.
References
1. Tierklinik Lüsche GmbH, Lüsche, Germany


