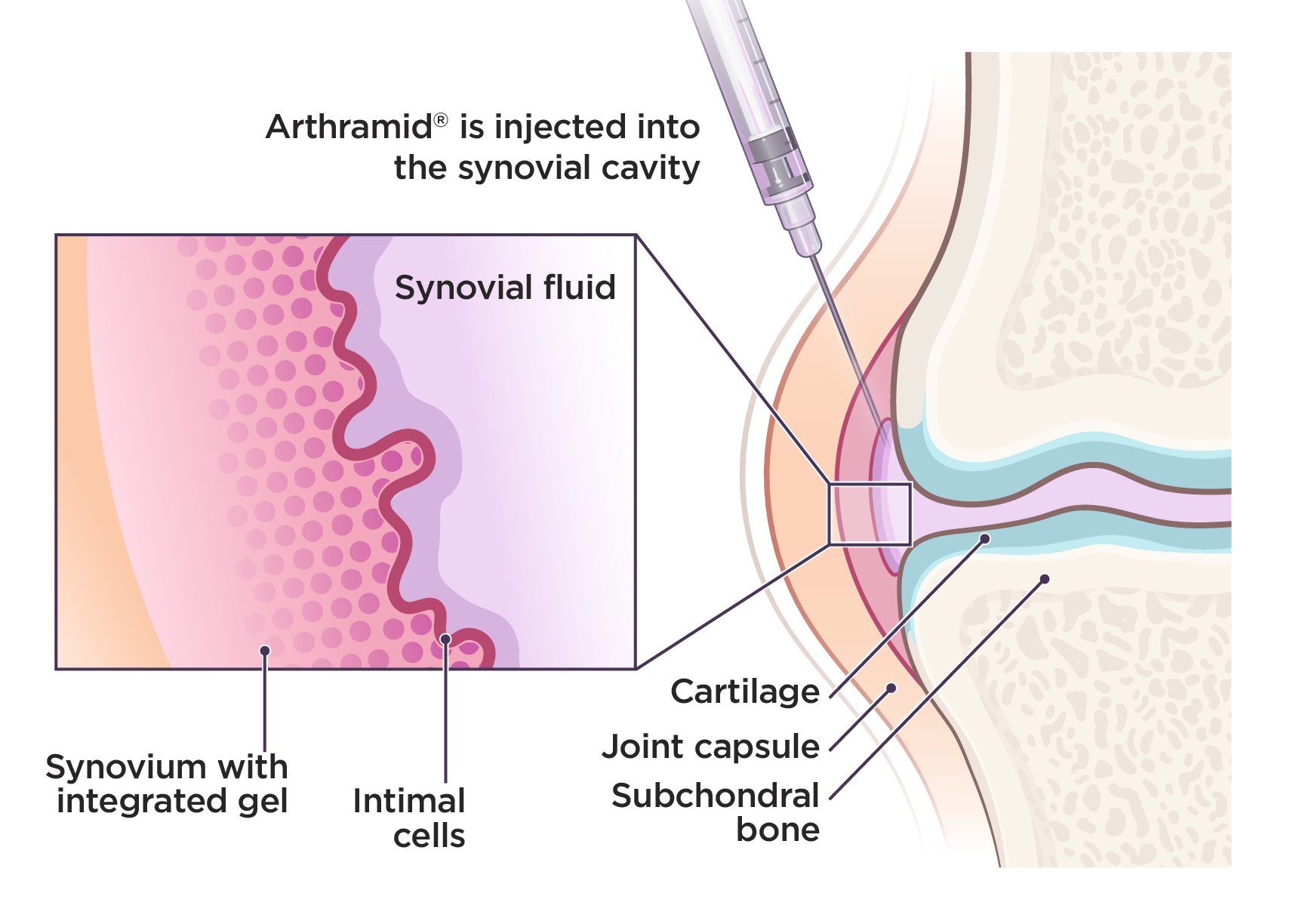
Arthramid is a patented 2.5% injectable polyacrylamide hydrogel (iPAAG). Administered through intra-articular joint injection, it is integrated into the synovium of the joint, improving function and resolving lameness.
Arthramid goes beyond conventional therapies to safely and sustainably manage osteoarthritis.
- Increased load transfer capacity through the joint capsule
- Reduced effusion and decreased stiffness
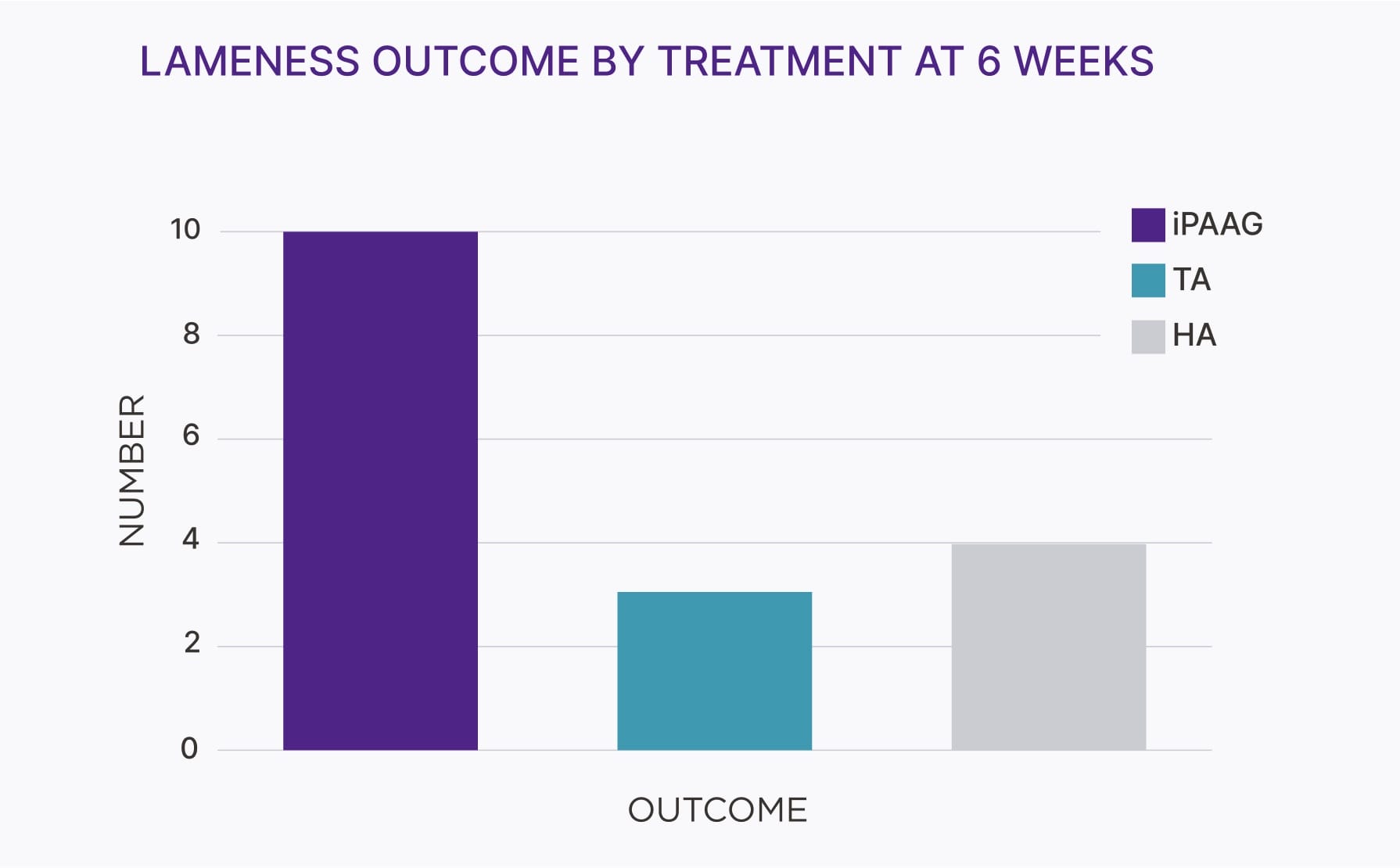
- Over 80% of patients remain lame-free after one injection